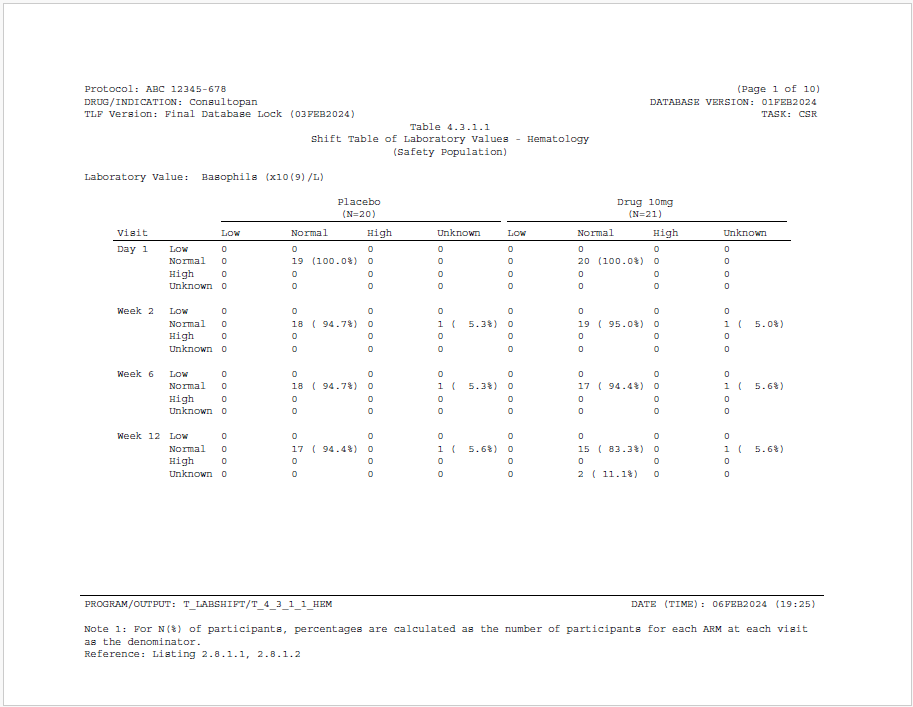
This example produces a shift table of lab values. A shift table is used to observe the change in lab values over visits, and is commonly used in the pharmaceutical industry.
Program
library(sassy)
options("logr.notes" = FALSE,
"logr.autolog" = TRUE,
"procs.print" = FALSE)
# Get temp location for log and report output
tmp <- tempdir()
lf <- log_open(file.path(tmp, "example16.log"))
# Get data ----------------------------------------------------------------
sep("Get data")
# Get sample data path
pth <- system.file("extdata", package = "sassy")
put("Open data library")
libname(sdtm, pth, "csv")
# Create Formats ----------------------------------------------------------
sep("Create Formats")
put("Format for visits")
vfmt <- value(condition(x == "DAY 1", "Day 1"),
condition(x == "WEEK 2", "Week 2"),
condition(x == "WEEK 6", "Week 6"),
condition(x == "WEEK 12", "Week 12"),
as.factor = TRUE)
put("Format for ARMs")
afmt <- value(condition(x == "ARM A", "Placebo"),
condition(x == "ARM B", "Drug (10mg)"),
condition(x == "ARM C", "Drug (20mg)"),
condition(x == "ARM D", "Competitor"),
as.factor = TRUE)
put("Format for Lab Result Indicator")
rfmt <- value(condition(x == "LOW", "Low"),
condition(x == "NORMAL", "Normal"),
condition(x == "HIGH", "High"),
condition(x == "UNKNOWN", "Unknown"),
as.factor = TRUE)
# Prepare data ------------------------------------------------------------
sep("Prepare data")
put("Pull out needed visits and columns")
lbsub1 <- subset(sdtm$LB, VISIT %in% toupper(levels(vfmt)),
v(USUBJID, VISIT, VISITNUM, LBCAT, LBORRESU, LBTEST,
LBTESTCD, LBBLFL, LBNRIND)) |> put()
put("Pull out baseline subset")
lbsub2 <- subset(lbsub1, LBBLFL == 'Y',
v(USUBJID, VISIT, LBCAT, LBTESTCD, LBNRIND)) |> put()
put("Merge and append change from baseline")
datastep(lbsub1, merge = lbsub2, merge_by = v(USUBJID, LBCAT, LBTESTCD),
rename = v(LBNRIND.1 = LBNRIND, LBNRIND.2 = BLBNRIND, VISIT.1 = VISIT),
drop = VISIT.2, {
if (is.na(LBNRIND.1)) {
LBNRIND.1 <- "UNKNOWN"
}
if (is.na(LBNRIND.2)) {
LBNRIND.2 <- "UNKNOWN"
}
}) -> lbsub
put("Pull needed ARMs and columns for DM")
dmsub <- subset(sdtm$DM, ARM != "SCREEN FAILURE" & is.na(ARM) == FALSE,
v(USUBJID, ARMCD, ARM)) |> put()
put("Merge DM with LB to get subject treatments")
datastep(lbsub, merge = dmsub, merge_by = USUBJID,
where = expression(toupper(VISIT) != 'SCREENING'),
{
VISIT <- fapply(VISIT, vfmt)
}) -> lbdat
# Get population counts ---------------------------------------------------
sep("Get population counts")
proc_sort(lbdat, by = v(ARM, USUBJID),
keep = v(ARM, USUBJID),
options = nodupkey) -> lb_unique
put("Get population frequencies")
proc_freq(lb_unique, tables = ARM,
output = long,
options = v(nopercent, nonobs)) -> lb_pop
# Prepare lab test labels -------------------------------------------------
sep("Lab test labels")
put("Get lookup data for lab tests")
proc_sort(lbdat, by = v(LBTESTCD, LBTEST, LBORRESU),
keep = v(LBTESTCD, LBTEST, LBORRESU),
options = nodupkey) -> tcodes
put("Create test label with units")
datastep(tcodes, where = expression(is.na(LBORRESU) == FALSE),
keep = v(LBTESTCD, LABEL),
{
LABEL <- paste0(LBTEST, " (", LBORRESU, ")")
}) -> tfmtdat
put("Create lab value lookup")
tfmt <- tfmtdat$LABEL
names(tfmt) <- tfmtdat$LBTESTCD
# Calculate frequencies ----------------------------------------------------
sep("Calculate frequencies")
put("Apply formats")
lbdat$LBNRIND <- fapply(lbdat$LBNRIND, rfmt)
lbdat$BLBNRIND <- fapply(lbdat$BLBNRIND, rfmt)
put("Get freqs by ARM and visit")
proc_freq(lbdat, by = v(ARM, LBTESTCD, VISIT),
tables = LBNRIND * BLBNRIND) -> lb_freq
put("Combine frequencies and percents")
datastep(lb_freq,
drop = v(VAR1, VAR2, CNT, PCT),
{
if (CNT == 0) {
CNTPCT <- fapply(CNT, "%d", width = 10, justify = "left")
} else {
CNTPCT <- fapply2(CNT, PCT, "%d", "(%5.1f%%)")
}
}) -> lb_comb
put("Transpose ARMs")
proc_transpose(lb_comb, id = v(BY1, CAT2), copy = N,
by = v(BY2, BY3, CAT1), var = CNTPCT,
options = noname) -> lb_final
put("Apply formats")
lb_final$BY2 <- fapply(lb_final$BY2, tfmt)
put("Rename variables")
datastep(lb_final,
rename = c(BY2 = "LBTEST", BY3 = "VISIT", CAT1 = "RIND"),
{}) -> lb_final
put("Sort by lab test")
proc_sort(lb_final, by = v(LBTEST, VISIT)) -> lb_final
# Create report -----------------------------------------------------------
sep("Create report")
put("Create output path")
pth <- file.path(tmp, "output/example16.pdf") |> put()
tbl <- create_table(lb_final) |>
spanning_header(`ARM A.Low`, `ARM A.Unknown`, "Placebo", n = lb_pop["ARM A"]) |>
spanning_header(`ARM B.Low`, `ARM B.Unknown`, "Drug 10mg", n = lb_pop["ARM B"]) |>
spanning_header(`ARM C.Low`, `ARM C.Unknown`, "Drug 20mg", n = lb_pop["ARM C"]) |>
spanning_header(`ARM D.Low`, `ARM D.Unknown`, "Competitor", n = lb_pop["ARM D"]) |>
define(LBTEST, visible = FALSE) |>
define(VISIT, "Visit", format = vfmt, dedupe = TRUE, align = "left",
id_var = TRUE, blank_after = TRUE) |>
define(N, "n", visible = FALSE) |>
define(RIND, "", align = "left", id_var = TRUE) |>
define(`ARM A.Low`, "Low") |>
define(`ARM A.Normal`, "Normal") |>
define(`ARM A.High`, "High") |>
define(`ARM A.Unknown`, "Unknown") |>
define(`ARM B.Low`, "Low") |>
define(`ARM B.Normal`, "Normal") |>
define(`ARM B.High`, "High") |>
define(`ARM B.Unknown`, "Unknown") |>
define(`ARM C.Low`, "Low", page_wrap = TRUE) |>
define(`ARM C.Normal`, "Normal") |>
define(`ARM C.High`, "High") |>
define(`ARM C.Unknown`, "Unknown") |>
define(`ARM D.Low`, "Low") |>
define(`ARM D.Normal`, "Normal") |>
define(`ARM D.High`, "High") |>
define(`ARM D.Unknown`, "Unknown") |>
put("Create report")
rpt <- create_report(pth, output_type = "PDF",
font = "Courier", font_size = 9) |>
set_margins(top = 1, left = 1, right = 1, bottom = .5) |>
page_header(c("Protocol: ABC 12345-678", "DRUG/INDICATION: Consultopan",
"TLF Version: Final Database Lock (03FEB2024)"),
right = c("(Page [pg] of [tpg])", "DATABASE VERSION: 01FEB2024",
"TASK: CSR")) |>
titles("Table 4.3.1.1", "", "Shift Table of Laboratory Values - Hematology",
"(Safety Population)", blank_row = "below") |>
page_by(LBTEST, "Laboratory Value: ") |>
add_content(tbl) |>
footnotes("PROGRAM/OUTPUT: T_LABSHIFT/T_4_3_1_1_HEM",
"DATE (TIME): " %p% toupper(fapply(Sys.time(), "%d%b%Y (%H:%M)")),
columns = 2, borders = "top", blank_row = "below") |>
footnotes(paste("Note 1: For N(%) of participants, percentages are calculated",
"as the number of participants for each ARM at each visit",
"as the denominator."),
"Reference: Listing 2.8.1.1, 2.8.1.2", blank_row = "none")
put("Write out report to file system")
res <- write_report(rpt)
# Clean Up ----------------------------------------------------------------
sep("Clean Up")
log_close()
# View report
# file.show(res$modified_path)
# View log
# file.show(lf)Log
Here is the log:
=========================================================================
Log Path: C:/Users/dbosa/AppData/Local/Temp/RtmpaGlETW/log/example16.log
Program Path: C:/packages/Testing/sassytests/ShiftTable2.R
Working Directory: C:/packages/Testing
User Name: dbosa
R Version: 4.3.2 (2023-10-31 ucrt)
Machine: SOCRATES x86-64
Operating System: Windows 10 x64 build 22621
Base Packages: stats graphics grDevices utils datasets methods base Other
Packages: tidylog_1.0.2 procs_1.0.5
reporter_1.4.3 libr_1.2.9 logr_1.3.5 fmtr_1.6.2 common_1.1.1 sassy_1.2.1
Log Start Time: 2024-02-06 14:52:04.476925
=========================================================================
=========================================================================
Get data
=========================================================================
Open data library
# library 'sdtm': 8 items
- attributes: csv not loaded
- path: C:/Users/dbosa/AppData/Local/R/win-library/4.4/sassy/extdata
- items:
Name Extension Rows Cols Size LastModified
1 AE csv 150 27 88.8 Kb 2024-05-03 08:12:15
2 DM csv 87 24 45.8 Kb 2024-05-03 08:12:15
3 DS csv 174 9 34.4 Kb 2024-05-03 08:12:15
4 EX csv 84 11 26.7 Kb 2024-05-03 08:12:15
5 IE csv 2 14 13.7 Kb 2024-05-03 08:12:15
6 LB csv 1618 24 348.7 Kb 2024-05-03 08:12:15
7 SV csv 685 10 70.6 Kb 2024-05-03 08:12:15
8 VS csv 3358 17 467.7 Kb 2024-05-03 08:12:15
=========================================================================
Create Formats
=========================================================================
Format for visits
# A user-defined format: 4 conditions
- as.factor: TRUE
Name Type Expression Label Order
1 obj U x == "DAY 1" Day 1 NA
2 obj U x == "WEEK 2" Week 2 NA
3 obj U x == "WEEK 6" Week 6 NA
4 obj U x == "WEEK 12" Week 12 NA
Format for ARMs
# A user-defined format: 4 conditions
- as.factor: TRUE
Name Type Expression Label Order
1 obj U x == "ARM A" Placebo NA
2 obj U x == "ARM B" Drug (10mg) NA
3 obj U x == "ARM C" Drug (20mg) NA
4 obj U x == "ARM D" Competitor NA
Format for Lab Result Indicator
# A user-defined format: 4 conditions
- as.factor: TRUE
Name Type Expression Label Order
1 obj U x == "LOW" Low NA
2 obj U x == "NORMAL" Normal NA
3 obj U x == "HIGH" High NA
4 obj U x == "UNKNOWN" Unknown NA
=========================================================================
Prepare data
=========================================================================
Pull out needed visits and columns
# A tibble: 1,578 × 9
USUBJID VISIT VISITNUM LBCAT LBORRESU LBTEST LBTESTCD LBBLFL LBNRIND
<chr> <chr> <dbl> <chr> <chr> <chr> <chr> <chr> <chr>
1 ABC-01-049 DAY 1 1 HEMATOLOGY x10(9)/L Basophils BASO Y NORMAL
2 ABC-01-049 WEEK 2 2 HEMATOLOGY x10(9)/L Basophils BASO <NA> NORMAL
3 ABC-01-049 WEEK 6 6 HEMATOLOGY x10(9)/L Basophils BASO <NA> NORMAL
4 ABC-01-049 WEEK 12 12 HEMATOLOGY x10(9)/L Basophils BASO <NA> NORMAL
5 ABC-01-049 DAY 1 1 HEMATOLOGY x10(9)/L Eosinophi… EOS Y NORMAL
6 ABC-01-049 WEEK 2 2 HEMATOLOGY x10(9)/L Eosinophi… EOS <NA> NORMAL
7 ABC-01-049 WEEK 6 6 HEMATOLOGY x10(9)/L Eosinophi… EOS <NA> NORMAL
8 ABC-01-049 WEEK 12 12 HEMATOLOGY x10(9)/L Eosinophi… EOS <NA> NORMAL
9 ABC-01-049 DAY 1 1 HEMATOLOGY % Hematocrit HCT Y NORMAL
10 ABC-01-049 WEEK 2 2 HEMATOLOGY % Hematocrit HCT <NA> NORMAL
# ℹ 1,568 more rows
# ℹ Use `print(n = ...)` to see more rows
Pull out baseline subset
# A tibble: 409 × 5
USUBJID VISIT LBCAT LBTESTCD LBNRIND
<chr> <chr> <chr> <chr> <chr>
1 ABC-01-049 DAY 1 HEMATOLOGY BASO NORMAL
2 ABC-01-049 DAY 1 HEMATOLOGY EOS NORMAL
3 ABC-01-049 DAY 1 HEMATOLOGY HCT NORMAL
4 ABC-01-049 DAY 1 HEMATOLOGY HGB NORMAL
5 ABC-01-049 DAY 1 HEMATOLOGY LYM NORMAL
6 ABC-01-050 DAY 1 HEMATOLOGY BASO NORMAL
7 ABC-01-050 DAY 1 HEMATOLOGY EOS NORMAL
8 ABC-01-050 DAY 1 HEMATOLOGY HCT NORMAL
9 ABC-01-050 DAY 1 HEMATOLOGY HGB NORMAL
10 ABC-01-050 DAY 1 HEMATOLOGY LYM NORMAL
# ℹ 399 more rows
# ℹ Use `print(n = ...)` to see more rows
Merge and append change from baseline
datastep: columns increased from 9 to 10
# A tibble: 1,578 × 10
USUBJID VISIT VISITNUM LBCAT LBORRESU LBTEST LBTESTCD LBBLFL LBNRIND BLBNRIND
<chr> <chr> <dbl> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
1 ABC-01-049 WEEK 12 12 HEMA… x10(9)/L Basop… BASO <NA> NORMAL NORMAL
2 ABC-01-049 WEEK 2 2 HEMA… x10(9)/L Basop… BASO <NA> NORMAL NORMAL
3 ABC-01-049 DAY 1 1 HEMA… x10(9)/L Basop… BASO Y NORMAL NORMAL
4 ABC-01-049 WEEK 6 6 HEMA… x10(9)/L Basop… BASO <NA> NORMAL NORMAL
5 ABC-01-049 WEEK 12 12 HEMA… x10(9)/L Eosin… EOS <NA> NORMAL NORMAL
6 ABC-01-049 DAY 1 1 HEMA… x10(9)/L Eosin… EOS Y NORMAL NORMAL
7 ABC-01-049 WEEK 2 2 HEMA… x10(9)/L Eosin… EOS <NA> NORMAL NORMAL
8 ABC-01-049 WEEK 6 6 HEMA… x10(9)/L Eosin… EOS <NA> NORMAL NORMAL
9 ABC-01-049 WEEK 12 12 HEMA… % Hemat… HCT <NA> NORMAL NORMAL
10 ABC-01-049 WEEK 2 2 HEMA… % Hemat… HCT <NA> NORMAL NORMAL
# ℹ 1,568 more rows
# ℹ Use `print(n = ...)` to see more rows
Pull needed ARMs and columns for DM
# A tibble: 85 × 3
USUBJID ARMCD ARM
<chr> <chr> <chr>
1 ABC-01-049 4 ARM D
2 ABC-01-050 2 ARM B
3 ABC-01-051 1 ARM A
4 ABC-01-052 3 ARM C
5 ABC-01-053 2 ARM B
6 ABC-01-054 4 ARM D
7 ABC-01-055 3 ARM C
8 ABC-01-056 1 ARM A
9 ABC-01-113 4 ARM D
10 ABC-01-114 2 ARM B
# ℹ 75 more rows
# ℹ Use `print(n = ...)` to see more rows
Merge DM with LB to get subject treatments
datastep: columns increased from 10 to 12
# A tibble: 1,578 × 12
USUBJID VISIT VISITNUM LBCAT LBORRESU LBTEST LBTESTCD LBBLFL LBNRIND BLBNRIND
<chr> <ord> <dbl> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
1 ABC-01-049 Week 12 12 HEMA… x10(9)/L Lymph… LYM <NA> NORMAL NORMAL
2 ABC-01-049 Week 6 6 HEMA… x10(9)/L Lymph… LYM <NA> NORMAL NORMAL
3 ABC-01-049 Week 2 2 HEMA… x10(9)/L Lymph… LYM <NA> NORMAL NORMAL
4 ABC-01-049 Week 6 6 HEMA… x10(9)/L Basop… BASO <NA> NORMAL NORMAL
5 ABC-01-049 Week 12 12 HEMA… x10(9)/L Eosin… EOS <NA> NORMAL NORMAL
6 ABC-01-049 Week 12 12 HEMA… g/dL Hemog… HGB <NA> NORMAL NORMAL
7 ABC-01-049 Week 2 2 HEMA… x10(9)/L Eosin… EOS <NA> NORMAL NORMAL
8 ABC-01-049 Week 6 6 HEMA… x10(9)/L Eosin… EOS <NA> NORMAL NORMAL
9 ABC-01-049 Week 12 12 HEMA… % Hemat… HCT <NA> NORMAL NORMAL
10 ABC-01-049 Day 1 1 HEMA… x10(9)/L Lymph… LYM Y NORMAL NORMAL
# ℹ 1,568 more rows
# ℹ 2 more variables: ARMCD <chr>, ARM <chr>
# ℹ Use `print(n = ...)` to see more rows
=========================================================================
Get population counts
=========================================================================
proc_sort: input data set 86 rows and 12 columns
by: ARM USUBJID
keep: ARM USUBJID
order: a a
options: nodupkey
output data set 86 rows and 2 columns
# A tibble: 86 × 2
ARM USUBJID
<chr> <chr>
1 ARM A ABC-01-051
2 ARM A ABC-01-056
3 ARM A ABC-02-034
4 ARM A ABC-02-038
5 ARM A ABC-02-109
6 ARM A ABC-03-002
7 ARM A ABC-03-006
8 ARM A ABC-03-091
9 ARM A ABC-04-075
10 ARM A ABC-04-080
# ℹ 76 more rows
# ℹ Use `print(n = ...)` to see more rows
Get population frequencies
proc_freq: input data set 86 rows and 2 columns
tables: ARM
output: long
view: TRUE
output: 1 datasets
# A tibble: 1 × 6
VAR STAT `ARM A` `ARM B` `ARM C` `ARM D`
<chr> <chr> <dbl> <dbl> <dbl> <dbl>
1 ARM CNT 20 21 20 23
=========================================================================
Lab test labels
=========================================================================
Get lookup data for lab tests
proc_sort: input data set 10 rows and 12 columns
by: LBTESTCD LBTEST LBORRESU
keep: LBTESTCD LBTEST LBORRESU
order: a a a
options: nodupkey
output data set 10 rows and 3 columns
# A tibble: 10 × 3
LBTESTCD LBTEST LBORRESU
<chr> <chr> <chr>
1 BASO Basophils x10(9)/L
2 BASO Basophils <NA>
3 EOS Eosinophils x10(9)/L
4 EOS Eosinophils <NA>
5 HCT Hematocrit %
6 HCT Hematocrit <NA>
7 HGB Hemoglobin g/dL
8 HGB Hemoglobin <NA>
9 LYM Lymphocytes x10(9)/L
10 LYM Lymphocytes <NA>
Create test label with units
datastep: columns decreased from 3 to 2
# A tibble: 5 × 2
LBTESTCD LABEL
<chr> <chr>
1 BASO Basophils (x10(9)/L)
2 EOS Eosinophils (x10(9)/L)
3 HCT Hematocrit (%)
4 HGB Hemoglobin (g/dL)
5 LYM Lymphocytes (x10(9)/L)
Create lab value lookup
=========================================================================
Calculate frequencies
=========================================================================
Apply formats
Get freqs by ARM and visit
proc_freq: input data set 1578 rows and 12 columns
tables: LBNRIND * BLBNRIND
by: ARM LBTESTCD VISIT
view: TRUE
output: 1 datasets
# A tibble: 1,280 × 10
BY1 BY2 BY3 VAR1 VAR2 CAT1 CAT2 N CNT PCT
<chr> <chr> <ord> <chr> <chr> <fct> <fct> <dbl> <dbl> <dbl>
1 ARM A BASO Day 1 LBNRIND BLBNRIND Low Low 19 0 0
2 ARM A BASO Day 1 LBNRIND BLBNRIND Low Normal 19 0 0
3 ARM A BASO Day 1 LBNRIND BLBNRIND Low High 19 0 0
4 ARM A BASO Day 1 LBNRIND BLBNRIND Low Unknown 19 0 0
5 ARM A BASO Day 1 LBNRIND BLBNRIND Normal Low 19 0 0
6 ARM A BASO Day 1 LBNRIND BLBNRIND Normal Normal 19 19 100
7 ARM A BASO Day 1 LBNRIND BLBNRIND Normal High 19 0 0
8 ARM A BASO Day 1 LBNRIND BLBNRIND Normal Unknown 19 0 0
9 ARM A BASO Day 1 LBNRIND BLBNRIND High Low 19 0 0
10 ARM A BASO Day 1 LBNRIND BLBNRIND High Normal 19 0 0
# ℹ 1,270 more rows
# ℹ Use `print(n = ...)` to see more rows
Combine frequencies and percents
datastep: columns decreased from 10 to 7
# A tibble: 1,280 × 7
BY1 BY2 BY3 CAT1 CAT2 N CNTPCT
<chr> <chr> <ord> <fct> <fct> <dbl> <chr>
1 ARM A BASO Day 1 Low Low 19 "0 "
2 ARM A BASO Day 1 Low Normal 19 "0 "
3 ARM A BASO Day 1 Low High 19 "0 "
4 ARM A BASO Day 1 Low Unknown 19 "0 "
5 ARM A BASO Day 1 Normal Low 19 "0 "
6 ARM A BASO Day 1 Normal Normal 19 "19 (100.0%)"
7 ARM A BASO Day 1 Normal High 19 "0 "
8 ARM A BASO Day 1 Normal Unknown 19 "0 "
9 ARM A BASO Day 1 High Low 19 "0 "
10 ARM A BASO Day 1 High Normal 19 "0 "
# ℹ 1,270 more rows
# ℹ Use `print(n = ...)` to see more rows
Transpose ARMs
proc_transpose: input data set 1280 rows and 7 columns
by: BY2 BY3 CAT1
var: CNTPCT
id: BY1 CAT2
copy: N
name: NAME
output dataset 80 rows and 20 columns
# A tibble: 80 × 20
BY2 BY3 CAT1 N `ARM A.Low` `ARM A.Normal` `ARM A.High` `ARM A.Unknown`
<chr> <ord> <fct> <dbl> <chr> <chr> <chr> <chr>
1 BASO Day 1 Low 19 "0 " "0 " "0 " "0 "
2 EOS Day 1 Low 19 "3 ( 15.8%)" "0 " "0 " "0 "
3 HCT Day 1 Low 20 "3 ( 15.0%)" "0 " "0 " "0 "
4 HGB Day 1 Low 20 "3 ( 15.0%)" "0 " "0 " "0 "
5 LYM Day 1 Low 19 "0 " "0 " "0 " "0 "
6 BASO Week 2 Low 19 "0 " "0 " "0 " "0 "
7 EOS Week 2 Low 19 "1 ( 5.3%)" "0 " "0 " "0 "
8 HCT Week 2 Low 20 "1 ( 5.0%)" "1 ( 5.0%)" "0 " "0 "
9 HGB Week 2 Low 20 "1 ( 5.0%)" "1 ( 5.0%)" "0 " "0 "
10 LYM Week 2 Low 19 "0 " "0 " "0 " "0 "
# ℹ 70 more rows
# ℹ 12 more variables: `ARM B.Low` <chr>, `ARM B.Normal` <chr>, `ARM B.High` <chr>,
# `ARM B.Unknown` <chr>, `ARM C.Low` <chr>, `ARM C.Normal` <chr>,
# `ARM C.High` <chr>, `ARM C.Unknown` <chr>, `ARM D.Low` <chr>,
# `ARM D.Normal` <chr>, `ARM D.High` <chr>, `ARM D.Unknown` <chr>
# ℹ Use `print(n = ...)` to see more rows
Apply formats
Rename variables
datastep: columns started with 20 and ended with 20
# A tibble: 80 × 20
LBTEST VISIT RIND N `ARM A.Low` `ARM A.Normal` `ARM A.High` `ARM A.Unknown`
<chr> <ord> <fct> <dbl> <chr> <chr> <chr> <chr>
1 Basophi… Day 1 Low 19 "0 … "0 " "0 " "0 "
2 Eosinop… Day 1 Low 19 "3 ( 15.8%… "0 " "0 " "0 "
3 Hematoc… Day 1 Low 20 "3 ( 15.0%… "0 " "0 " "0 "
4 Hemoglo… Day 1 Low 20 "3 ( 15.0%… "0 " "0 " "0 "
5 Lymphoc… Day 1 Low 19 "0 … "0 " "0 " "0 "
6 Basophi… Week… Low 19 "0 … "0 " "0 " "0 "
7 Eosinop… Week… Low 19 "1 ( 5.3%… "0 " "0 " "0 "
8 Hematoc… Week… Low 20 "1 ( 5.0%… "1 ( 5.0%)" "0 " "0 "
9 Hemoglo… Week… Low 20 "1 ( 5.0%… "1 ( 5.0%)" "0 " "0 "
10 Lymphoc… Week… Low 19 "0 … "0 " "0 " "0 "
# ℹ 70 more rows
# ℹ 12 more variables: `ARM B.Low` <chr>, `ARM B.Normal` <chr>, `ARM B.High` <chr>,
# `ARM B.Unknown` <chr>, `ARM C.Low` <chr>, `ARM C.Normal` <chr>,
# `ARM C.High` <chr>, `ARM C.Unknown` <chr>, `ARM D.Low` <chr>,
# `ARM D.Normal` <chr>, `ARM D.High` <chr>, `ARM D.Unknown` <chr>
# ℹ Use `print(n = ...)` to see more rows
Sort by lab test
proc_sort: input data set 80 rows and 20 columns
by: LBTEST VISIT
keep: LBTEST VISIT RIND N ARM A.Low ARM A.Normal ARM A.High ARM A.Unknown ARM B.Low ARM B.Normal ARM B.High ARM B.Unknown ARM C.Low ARM C.Normal ARM C.High ARM C.Unknown ARM D.Low ARM D.Normal ARM D.High ARM D.Unknown
order: a a
output data set 80 rows and 20 columns
# A tibble: 80 × 20
LBTEST VISIT RIND N `ARM A.Low` `ARM A.Normal` `ARM A.High` `ARM A.Unknown`
<chr> <ord> <fct> <dbl> <chr> <chr> <chr> <chr>
1 Basophi… Day 1 Low 19 "0 … "0 " "0 " "0 "
2 Basophi… Day 1 Norm… 19 "0 … "19 (100.0%)" "0 " "0 "
3 Basophi… Day 1 High 19 "0 … "0 " "0 " "0 "
4 Basophi… Day 1 Unkn… 19 "0 … "0 " "0 " "0 "
5 Basophi… Week… Low 19 "0 … "0 " "0 " "0 "
6 Basophi… Week… Norm… 19 "0 … "18 ( 94.7%)" "0 " "1 ( 5.3%)"
7 Basophi… Week… High 19 "0 … "0 " "0 " "0 "
8 Basophi… Week… Unkn… 19 "0 … "0 " "0 " "0 "
9 Basophi… Week… Low 19 "0 … "0 " "0 " "0 "
10 Basophi… Week… Norm… 19 "0 … "18 ( 94.7%)" "0 " "1 ( 5.3%)"
# ℹ 70 more rows
# ℹ 12 more variables: `ARM B.Low` <chr>, `ARM B.Normal` <chr>, `ARM B.High` <chr>,
# `ARM B.Unknown` <chr>, `ARM C.Low` <chr>, `ARM C.Normal` <chr>,
# `ARM C.High` <chr>, `ARM C.Unknown` <chr>, `ARM D.Low` <chr>,
# `ARM D.Normal` <chr>, `ARM D.High` <chr>, `ARM D.Unknown` <chr>
# ℹ Use `print(n = ...)` to see more rows
=========================================================================
Create report
=========================================================================
Create output path
C:\Users\dbosa\AppData\Local\Temp\RtmpaGlETW/output/example16.pdf
# A table specification:
- data: tibble 'lb_final' 80 rows 20 cols
- show_cols: all
- use_attributes: all
- spanning_header: from='ARM A.Low' to='ARM A.Unknown' 'Placebo' level=1
- spanning_header: from='ARM B.Low' to='ARM B.Unknown' 'Drug 10mg' level=1
- spanning_header: from='ARM C.Low' to='ARM C.Unknown' 'Drug 20mg' level=1
- spanning_header: from='ARM D.Low' to='ARM D.Unknown' 'Competitor' level=1
- define: LBTEST visible='FALSE'
- define: VISIT 'Visit' align='left' id_var='TRUE' dedupe='TRUE'
- define: N visible='FALSE'
- define: RIND '' align='left' id_var='TRUE'
- define: ARM A.Low 'Low'
- define: ARM A.Normal 'Normal'
- define: ARM A.High 'High'
- define: ARM A.Unknown 'Unknown'
- define: ARM B.Low 'Low'
- define: ARM B.Normal 'Normal'
- define: ARM B.High 'High'
- define: ARM B.Unknown 'Unknown'
- define: ARM C.Low 'Low' page_wrap='TRUE'
- define: ARM C.Normal 'Normal'
- define: ARM C.High 'High'
- define: ARM C.Unknown 'Unknown'
- define: ARM D.Low 'Low'
- define: ARM D.Normal 'Normal'
- define: ARM D.High 'High'
- define: ARM D.Unknown 'Unknown'
Write out report to file system
# A report specification: 10 pages
- file_path: 'C:\Users\dbosa\AppData\Local\Temp\RtmpaGlETW/output/example16.pdf'
- output_type: PDF
- units: inches
- orientation: landscape
- margins: top 1 bottom 0.5 left 1 right 1
- line size/count: 9/46
- page by: LBTEST
- page_header: left=Protocol: ABC 12345-678, DRUG/INDICATION: Consultopan, TLF Version: Final Database Lock (03FEB2024) right=(Page [pg] of [tpg]), DATABASE VERSION: 01FEB2024, TASK: CSR
- title 1: 'Table 4.3.1.1'
- title 2: ''
- title 3: 'Shift Table of Laboratory Values - Hematology'
- title 4: '(Safety Population)'
- footnote 1: 'PROGRAM/OUTPUT: T_LABSHIFT/T_4_3_1_1_HEM'
- footnote 2: 'DATE (TIME): 06FEB2024 (14:52)'
- footnote 3: 'Note 1: For N(%) of participants, percentages are calculated as the number of participants for each ARM at each visit as the denominator.'
- footnote 4: 'Reference: Listing 2.8.1.1, 2.8.1.2'
- content:
# A table specification:
- data: tibble 'lb_final' 80 rows 20 cols
- show_cols: all
- use_attributes: all
- spanning_header: from='ARM A.Low' to='ARM A.Unknown' 'Placebo' level=1
- spanning_header: from='ARM B.Low' to='ARM B.Unknown' 'Drug 10mg' level=1
- spanning_header: from='ARM C.Low' to='ARM C.Unknown' 'Drug 20mg' level=1
- spanning_header: from='ARM D.Low' to='ARM D.Unknown' 'Competitor' level=1
- define: LBTEST visible='FALSE'
- define: VISIT 'Visit' align='left' id_var='TRUE' dedupe='TRUE'
- define: N visible='FALSE'
- define: RIND '' align='left' id_var='TRUE'
- define: ARM A.Low 'Low'
- define: ARM A.Normal 'Normal'
- define: ARM A.High 'High'
- define: ARM A.Unknown 'Unknown'
- define: ARM B.Low 'Low'
- define: ARM B.Normal 'Normal'
- define: ARM B.High 'High'
- define: ARM B.Unknown 'Unknown'
- define: ARM C.Low 'Low' page_wrap='TRUE'
- define: ARM C.Normal 'Normal'
- define: ARM C.High 'High'
- define: ARM C.Unknown 'Unknown'
- define: ARM D.Low 'Low'
- define: ARM D.Normal 'Normal'
- define: ARM D.High 'High'
- define: ARM D.Unknown 'Unknown'
=========================================================================
Clean Up
=========================================================================
=========================================================================
Log End Time: 2024-02-06 14:52:16.546017
Log Elapsed Time: 0 00:00:12
=========================================================================